Technical Field
Synthag is the term of the process that produces clusters of silver ions (Ag ++) stable to light and at room temperature, soluble in water, with antibacterial and antifungal properties.
Furthermore, the Synthag process refers to the preparation of a supramolecular structure consisting of clusters of silver ions linked together with propylene glycol bridges.
The Synthag procedure also concerns the use of these clusters as antimicrobial agents for the production of formulations for the treatment or prevention of bacterial and fungal infections.
Pre-existing technique
In the art, biocides and antibiotics are known both of natural origin and of complete or partial synthesis. However, these have limited action spectra and their continuous use in the medical field has created resistant strains of bacteria, reducing their effectiveness.
The purpose of the present procedure is therefore to find an effective and preferable alternative in various situations to biocides and antibiotics known in the art for the treatment or prevention of bacterial and fungal infections, in the pharmaceutical, veterinary, agricultural, cosmetic, industrial fields. and coating (“coating”) in general.
Synthag Show
In a first aspect, the Synthag refers to clusters of Ag + silver ions.
In fact, the invention of Synthag stems from the general consideration according to which the technical problem highlighted above can be solved effectively and reliably by means of clusters of water-soluble Ag + silver ions, linked together by weak interactions (weak interactions mean in particular interactions interactions with energy similar to that of hydrogen bonds or external sphere coordination) with the hydroxyl groups of propylene glycol and / or other polymers with polar groups that act as a bridge between the two or more silver atoms, in which said clusters further comprise hydrogen peroxide.
In this way, the clusters of Ag + silver ions are stable to light and at room temperature, are soluble in water, and create weak bonds with the indicated hydroxyl groups forming complex polymeric structures. The clusters of silver Ag + ions, bound to polymers, have a strong antimicrobial activity against all bacteria, Gram-positive and Gram-negative, avoiding any type of drug resistance. The difference with antibiotics lies in the drug resistance and invasiveness of the latter.
Furthermore, the presence of hydrogen peroxide allows to stabilize the clusters of silver Ag + ions in the oxidation state +1 and to extend their shelf life. Therefore, their use represents a solution of antibacterial capacity (such as, for example, referred but not limited to bacteria of the type Enterococcus faecalis, Pseudomonas aeruginosa, Escherichia coli, etc.) and antifungal capacity (such as, for example, referred to a wide range of fungi, including, but not limited to, Candida albicans) free from possible toxicity in the use of the metal, unlike the particles of metallic silver Ag0.
In fact, the topical use of said clusters of silver Ag + ions directly on the infected surface, exploiting the low migration capacity of silver ions in the tissues and the low toxicity to the dermis, also brings benefits in the case of other inflammatory diseases caused by bacterial infections. In fact, the cause of the infection is eliminated, i.e. the presence and formation of bacterial colonies on the skin and internal tissues (nose, ears, throat, anus, vagina, etc.), using a special vehicle, such as cream, gel, ointment, aerosol, liquid mixture.
For example, in the case of candidiasis, the development of products based on clusters of silver Ag + ions against candida is a condition that has been treated with very positive developments.
In the present description, the term “cluster of Ag + silver ions” indicates a set of Ag + ions substantially bound with a polymeric matrix and molecules with more than one hydroxyl group capable of bridging two Ag + ions.
In the present description, the term “polymer” indicates a synthetic macromolecule comprising multiple repeating subunits.
In the present description, the term “polymer chain” indicates a polymer length comprising several subgroups connected together in the form of a chain.
According to a preferred embodiment, aforementioned polymers with polar groups are selected from the group which includes polyvinylpyrrolidone (PVP), polyacrylates, silica gel, polyols.
According to a preferred embodiment, aforementioned clusters of Ag + silver ions also include isopropyl alcohol, ethyl alcohol, citric acid, hyaluronic acid, iodine and / or other alcohols. In this way, said clusters of Ag + silver ions are stabilized and the antibacterial, antifungal and healing properties are also improved.
Furthermore, the aforementioned clusters of Ag + silver ions consist of various combinations and concentrations of propylene glycol, alcohols and said polymers with polar groups selected from the group that includes polyvinylpyrrolidone (PVP), polyacrylates, silica gel.
According to a preferred embodiment, said Ag + ions are linked together as shown in formula (1):
X-Ag+-Y- [-Ag+ -Y-Ag+ -]n –Y-Ag+ -X (1)
where X is an isopropyl alcohol molecule and Y is propylene glycol.
According to a preferred embodiment, the Ag + ions are bound together as shown in formula (2):
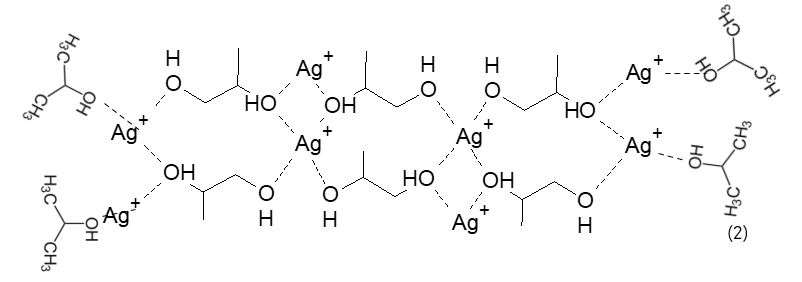
According to a preferred embodiment, the aforementioned clusters of Ag + silver ions have dimensions from about 10 nanometers up to over a thousand nanometers, depending on the synthesis conditions used.
It is thus possible to opt for the synthesis of nano-clusters (limit of nano particles, less than 100 nanometers) or of non-nano-particle clusters.
The ability to create clusters of Ag + silver ions, with dimensions greater than 100 nanometers, eliminates the problems due to the possible toxicity in the use of the metal.


